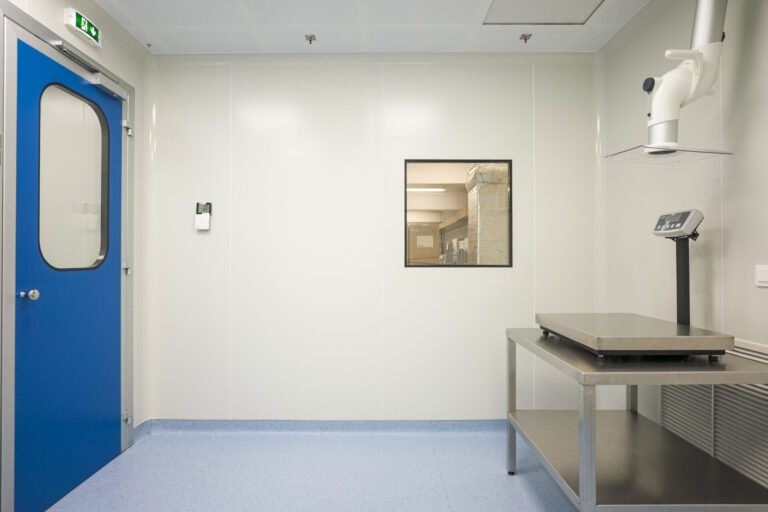
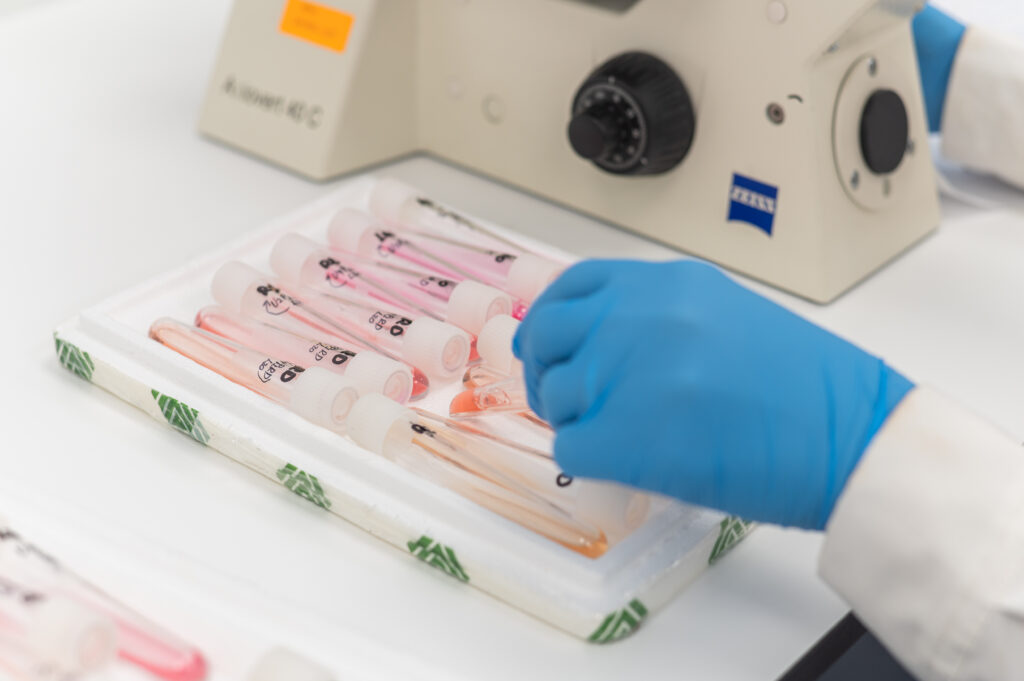
Cleanroom GMP Class A/B Re-Design & Construction for the Quality Control Department of the Hellenic Pasteur Institute. It is used as a laboratory for the quality control of injectables.
Laboratories
Athens

2020
Due to the nature of the product which enters directly into the human body, the purity conditions in such laboratories play an extremely important role in avoiding any kind of contamination.
The design, the construction materials, the HVAC system of the space require specialized knowledge certifications in Cleanroom Design, Construction & Validation which our company provides’.
Commissioning and Cleanroom Certification according to ISO 14644 & EU GMP Volume 4 Annex 1 totally executed by BCT Group specialists.